Do your product’s benefits outweigh its potential risks? More importantly, do you have the right data to credibly demonstrate this to regulators, payers, physicians, and patients?
You can rely on our experienced team to help you navigate the ever-evolving landscape of benefit-risk assessment and meet the requirements of regulatory and reimbursement stakeholders worldwide. Our integrated team of cross-functional experts can provide you with the guidance you need to synthesize, summarize, and confidently act on the relevant evidence for your product. We’ll help you build consensus within your organization to achieve a common understanding, increased transparency, and improved communication about your product’s benefit-risk profile.
A Structured Approach Tailored to Your Needs
Our teams have the expertise essential for developing a robust plan to assess benefits and risks. Once we have helped you develop a complete picture of your data, we can help you identify gaps to inform a strategy for possible new studies to include in your clinical development program and clarify appropriate post-approval activities. This includes:
- Understanding how a structured approach can improve your evidence generation and communication strategy
- Incorporating patient and/or provider preferences and patient perspectives
- Selecting the best metrics for measuring key benefits and risks that will resonate with providers and patients post-approval
- Identifying the best analytic approach for synthesizing clinical trial and/or real-world data
- Implementing additional risk minimization measures, and studies to evaluate their effectiveness
- Initiating postmarketing safety studies and/or comparative effectiveness studies
The Value of Proactive Implementation
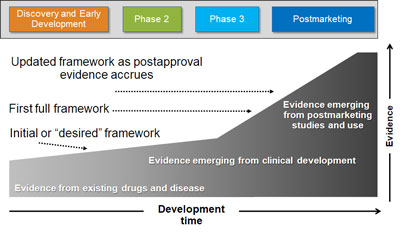
Conducting credible benefit-risk assessments presents unique challenges because the data on risks and benefits are not usually measured together in the same studies or study populations, and are not often gathered in a real-world setting. Additionally, these measurements might not be collected in the same way in both short and long-term studies or using the same units. By engaging with our team early in the development lifecycle, we can help you account for these challenges and avoid costly re-work and lost time at a critical stage in your product’s development process—while helping you position your product with strong benefit-risk evidence that resonates with your audience.




