A promising asset in development is just that—an exciting innovation with potential—until evidence of value has been generated. Creating a comprehensive health economic strategy to develop that evidence can help you minimize development time and costs and avoid duplication of efforts. The cornerstone of that plan is often an early cost-effectiveness model (CEM). These models provide an objective evidence-based framework to identify value drivers and associated evidence gaps and can inform an integrated evidence generation plan to gather necessary data to optimize future research investments.
The Advantages of an Early CRM
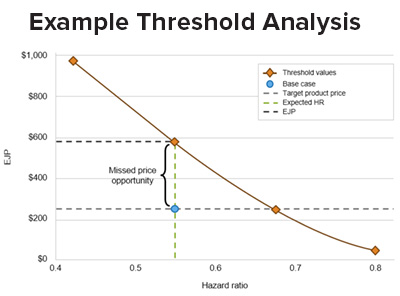 By facilitating extensive scenario analyses, an early CEM can help inform your:
By facilitating extensive scenario analyses, an early CEM can help inform your:
- target product profile (TPP)
- estimate economically justifiable price (EJP) and inform your value-based price strategy
- phase 2/3 clinical trial endpoints and study design
- target patient population(s)
- potential value arguments relative to alternative treatments.
Ultimately, the early CEM can serve as the foundation for the launch CEM you will need to support health technology assessment (HTA) submissions.
| Early Model | Launch model |
Context
| Context
|
Objectives
| Objectives
|
Dissemination
| Dissemination
|