Fully Portray the Clinical and Economic Value of Your Product
Contributed by:
Sorrel Wolowacz, PhD
Head, European Health Economics

We are often asked, “How can we assess whether EQ-5D is appropriate for estimating health utility in a particular patient population?” and “Will EQ-5D fully reflect the health-related quality-of-life improvement with a particular new product?”
Although all decision-makers accept EQ-5D data for health utility assessment in economic evaluations, it is widely recognised that it may not be appropriate for all health conditions. If there is any uncertainty, it is important to explore further before including it in a clinical trial or study.
Aspects to explore using empirical evidence if there is uncertainty about EQ-5D
Face validity – e.g., are there concepts included in conceptual models of health-related quality of life (HRQOL), patient-centred research, and/or validated condition-specific HRQOL measures that do not appear to be captured by the EQ-5D items (directly or indirectly)?
Does EQ-5D index decline with increasing severity of disease (as measured by a validated clinical measure)?
Does EQ-5D index improve on response to treatment (as demonstrated by a validated clinical measure)?
Is EQ-5D index lower for people with the condition compared with the general population (matched for age, sex and – if possible – comorbidity) where validated HRQOL measures (such as SF-36) demonstrate impairment in comparison with the general population?
Is there an association between EQ-5D index and a validated condition-specific HRQOL measure?
Alternative health utility assessment methods to EQ-5D
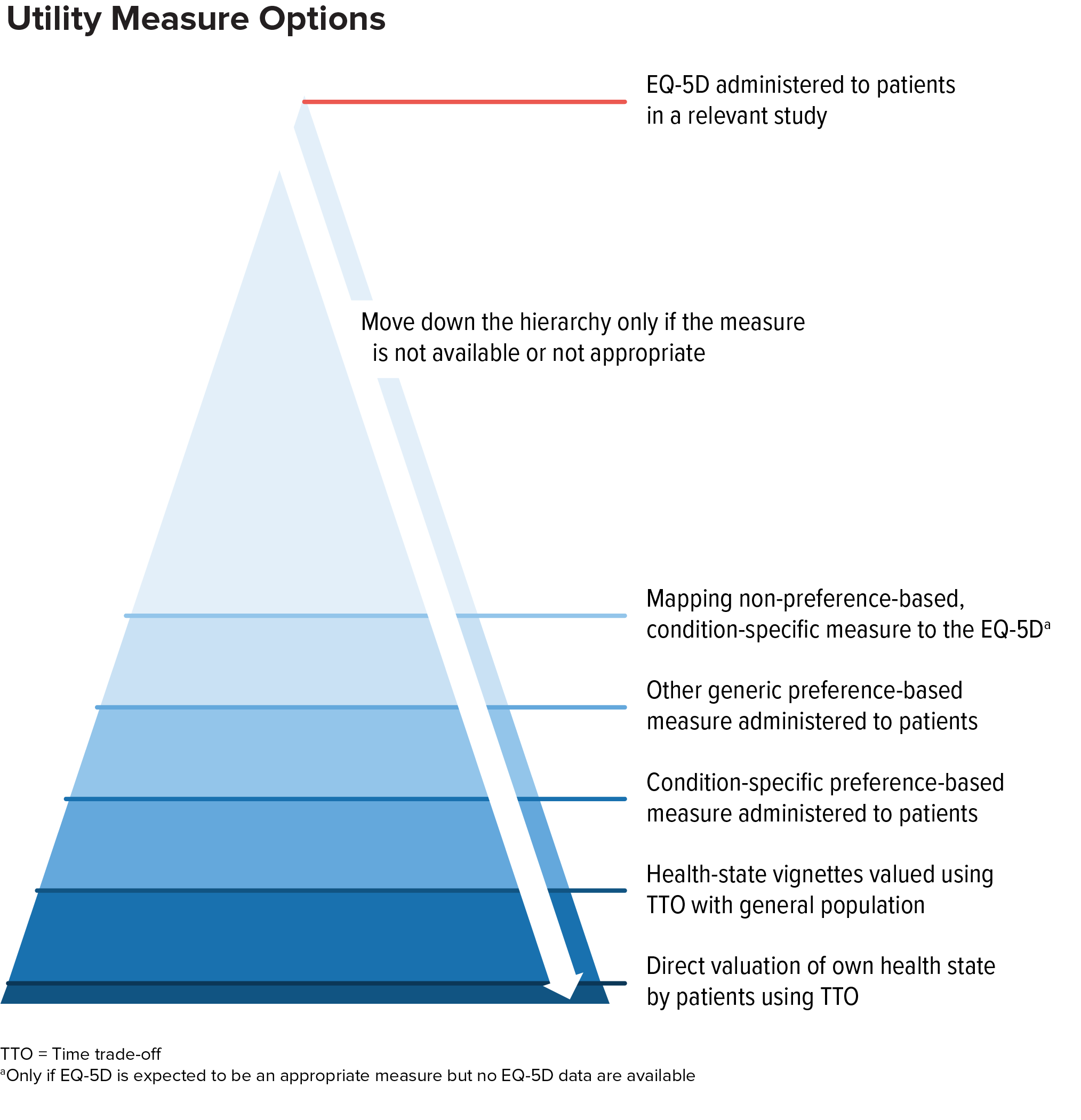
Alternatives to EQ-5D include other generic preference-based measures, condition-specific measures, mapping, direct valuation, and vignette valuation.

In general, decision-makers consider these alternatives as part of a hierarchy of evidence. If EQ-5D is not appropriate, other generic preference-based measures are typically considered the next-best alternative (provided a measure appropriate for the health condition is available).
Contact us for help in fully portraying the clinical and economic value of your product.
Generic utility measures differ quite considerably, for example in:
- the size and content of the descriptive system (dimensions, questions, and response levels)
- questionnaire length
- time to complete
- recall period
- modes of administration
- language versions
- method of valuation
Choosing the most appropriate measure can have an important impact on utility estimates and quality-adjusted life years gained in economic evaluations, and therefore the value-based price of a product. Careful selection is important for the development of high-quality utility estimates informing economic evaluations that affect patients’ access to treatments, physicians’ ability to use them, product price, and manufacturers return on investment.
Exploring Alternative Generic Preference-Based Utility Assessment Measures
Download our summary of Generic Preference-Based Health-Utility Measures and summary of Generic Preference-Based Measures for Children and Adolescents describing the characteristics of alternative generic preference-based utility measures to help you explore alternatives to EQ-5D. Reach out for help in determining whether EQ-5D is appropriate for your research (including collating empirical evidence to present to NICE), and which alternative measure or method may be most appropriate for your population of interest.
RTI-HS led the development of the ISPOR Good Research Practice Guideline for Health Utility Estimation in Clinical Studies (Wolowacz et al., Value Health 19(2016):704-19). doi: 10.1016/j.jval.2016.06.001 and offers a full range of services for health utility estimation, economic modeling and value communication.
In case you are wondering -
Why are Health Utility Data Important?
- Cost-utility analyses, most often performed in economic models, are increasingly used by HTA and pricing and reimbursement authorities in many countries to establish whether the cost of a new intervention can be justified in terms of expected health benefits.
- The decisions made by these authorities affect patients’ access to treatments, physicians’ ability to use them, the product’s price, and the return on investment that manufacturers are able to realise.
- Health-utility estimates are among the most important data inputs in cost-utility models; the accuracy and precision of the model results often depend heavily on the quality of the health state utility estimates.
