Pre-Approval Information Exchange (PIE) Materials
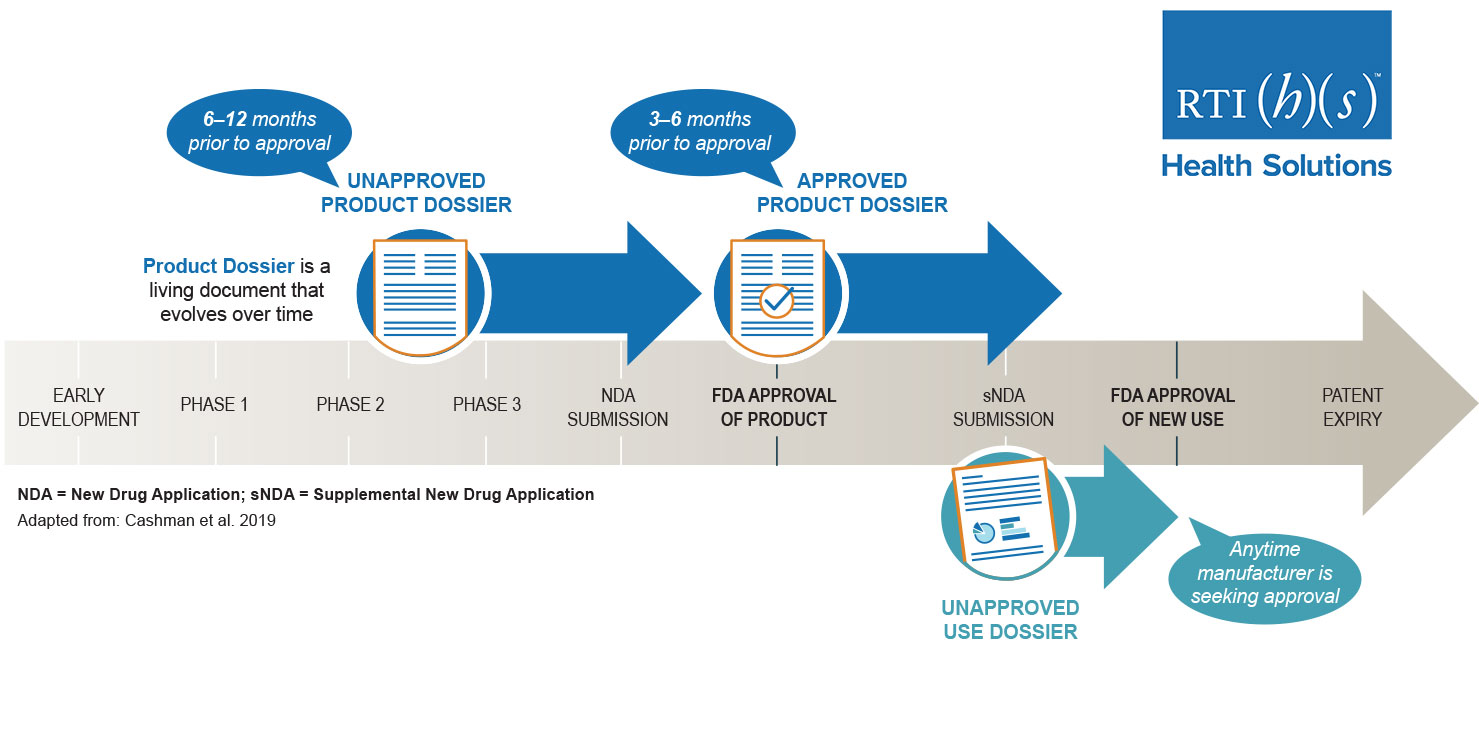
Unapproved Medical Product Dossiers and Unapproved Use Dossiers
An unapproved product dossier, or an unapproved use dossier, allows you to communicate key product information—including clinical evidence—to US payors well in advance of approval. Let our team of experts help you prepare dossiers to make the most of your pre-approval product communication opportunities. Key sections in these dossiers include:
- Highlights and Overview
- Product Information and Disease Description
- Clinical Evidence
- Economic Information
PIE Decks and Other Communication Materials
Sharing certain health care economic and scientific information about your products ahead of FDA approval can help improve patient access to emerging therapies and devices. Work with our team to create your PIE decks to help you share information such as burden of illness, unmet need, and early clinical data with stakeholders. We can also support your development of PIE webinars which allow your chosen clinical expert to present key disease and unmet need evidence to US health care decision makers. Getting this valuable information into the hands of decision makers as they are planning, forecasting, and budgeting for new pipeline products helps make new medications and devices available for patients as rapidly as possible.
AMCP Approved Product Dossiers
The AMCP format has been adopted by most health plans in the US as the standard for formulary submission dossiers. Our team of market access experts has extensive experience developing rigorous AMCP dossiers. You can confidently rely on us to prepare accurate, evidence-based, and scientifically focused materials to help you communicate your product’s key clinical and economic evidence to support your pricing, reimbursement, and formulary inclusion efforts. In addition to being used in response to unsolicited requests for information from payors, the information in an AMCP dossier can help you build trust with your customers and facilitate developing effective product marketing. Key sections include:
- Executive Summary
- Product Information and Disease Description
- Clinical Evidence
- Economic Value and Modeling Report
- Additional Supporting Evidence




